Build Regulatory Content with Confidence
Automate 70-80% of regulatory authoring.
Reduce submission time from months to weeks.
Across the drug lifecycle, regulatory teams manage expanding volumes of interdependent documents, where small inconsistencies introduce risk and cause significant downstream delays.
High-volume documentation to move from discovery to registration.
- IND packages (pre-cllinical, CMC, clinical plans).
- Protocols, amendments, IBs.
- CSRs, safety narratives, ISS/ISE.
- Early Module 2 and Module 3 summaries.
Complex submissions required to secure market authorization.
- Full CTD/eCTD dossiers (Modules 1–5).
- Country-specific variations and forms.
- HA queries, IRs, 483s, CRLs.
- Advisory committee briefing packages.
- eCTD publishing & technical validation.
Ongoing documentation to maintain global market access.
- Labeling updates and CMC variations.
- Renewals, supplements, market expansions.
- PSURs, PADERs, DSURs.
- Promotional and medical review packages.
- Registration maintenance & commitments.
Built to handle the hardest parts of Regulatory Authoring
Each feature is designed around real submission workflows, helping teams draft, review, and maintain dossiers with speed, accuracy, and confidence.
Built to handle the hardest parts of Regulatory Authoring
Each feature is designed around real submission workflows, helping teams draft, review, and maintain dossiers with speed, accuracy, and confidence.
Proven impact for regulatory teams.
Designed to meet the standards of high-stakes regulatory authoring.

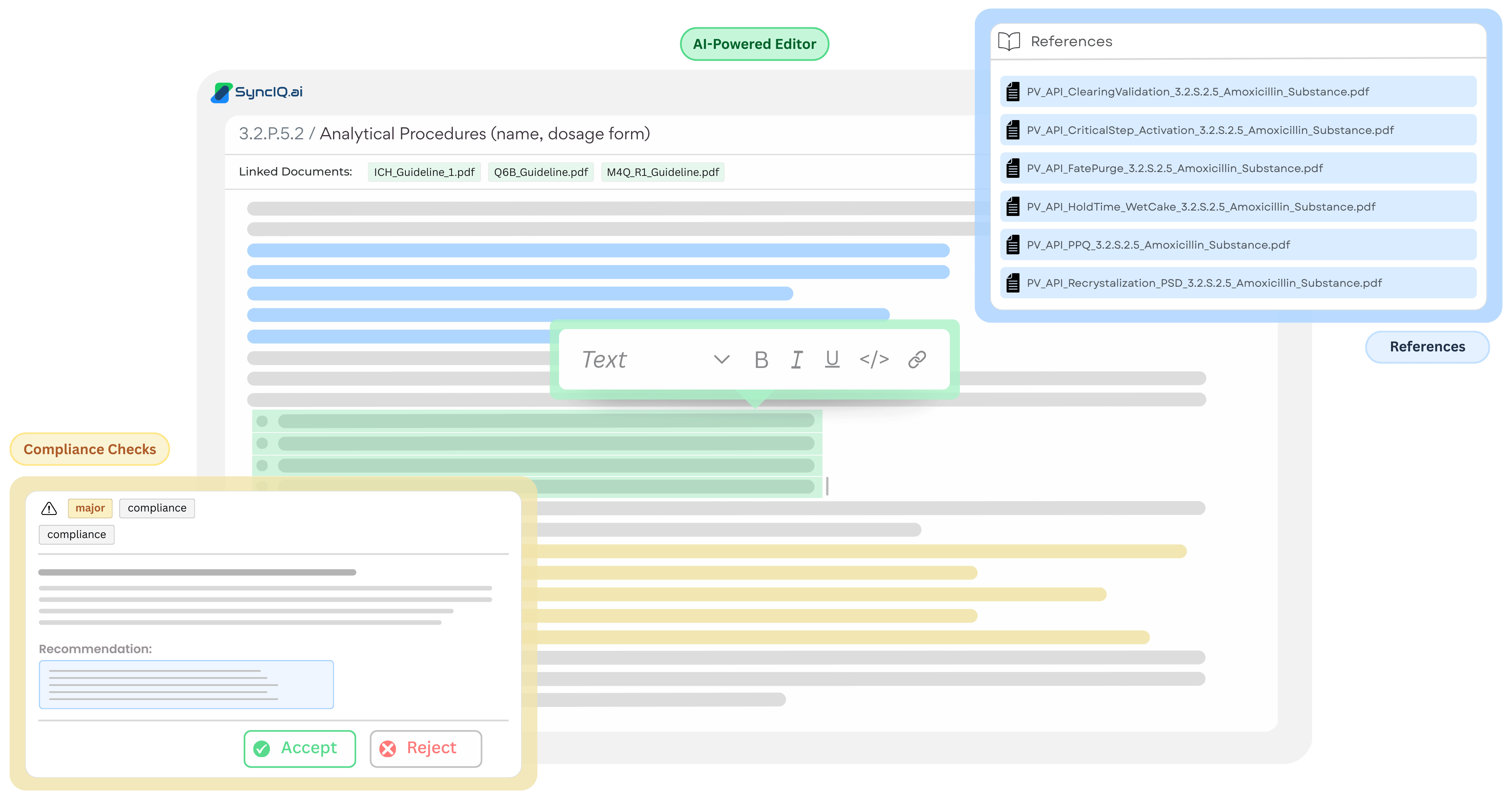
Fully traceable
All generated content maintains clear links to its original sources, enabling fast review, validation, and inspection-ready traceability.
Region-specific templates
Author content using predefined, region-aligned regulatory templates that guide structure, requirements, and formatting from the start.
Human in the loop
SyncIQ supports expert-led workflows where humans review, edit, and approve all content before updates.
Trusted by Regulatory, Clinical, and CMC teams worldwide
Set the pace for innovation in life sciences with SyncIQ
Learn how SyncIQ can help your organization file faster, scale smarter, and bring medical breakthroughs to patients months ahead of schedule.